Cosmetic products, being in direct human contact and touch, need to be safer than any other product category. Faulty manufacturing or unsafe use by consumers can cause severe contamination. Cosmetic products without preservatives are prone to microbial growth in their lifetime. Preservatives in cosmetic products ensure that they remain self-sterilizing towards growth of vegetative bacteria, yeasts and moulds and bacteriostatic or bactericidal for spores in all phases of their lifecycle: during production, packaging and usage. In cases when complete sterilization is impossible, at most they must ensure elimination of viable human pathogens at most and inhibitory action against residual non pathogens to the least.
We at HiMedia offer a complete range of products to achieve sterility for cosmetic products. Our media can ensure best sterility testing, total microbial count and detection of microbial presence.
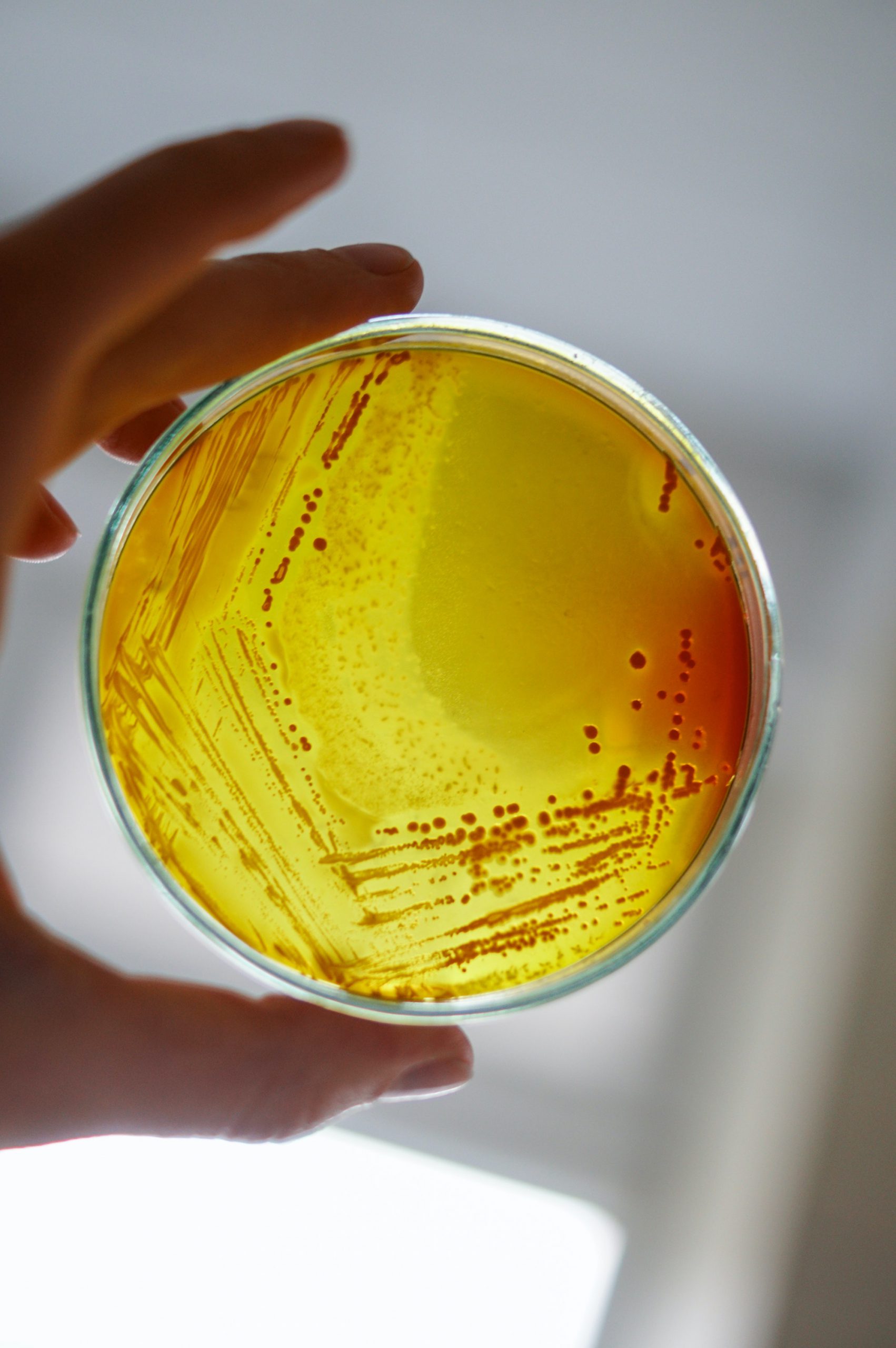
Microbial Detection Media
User safety is paramount in cosmetic products. Testing for presence of microbes and pathogens is very important for user safety. That is the reason why HiMedia provides various selective media for detection of specific microorganisms. The procedure is to dilute the cosmetic product and streak it onto the selective media. Later the plates are incubated. The checking is done for microbial growth of pathogens like Bacillus, Clostridia, Enterobacteriaceae, Lipolytic microorganisms, Staphylococci, Pseudomonas etc.
Sterility Testing
Metabolism of microorganisms can degrade and alter chemical composition of cosmetic products which can cause harm to customers. To avoid this, cosmetic products are put through sterility testing as a quality control measure with methods like direct colony counts and enrichment culturing for isolating microorganisms from cosmetic products. HiMedia provides various Culture Media which are recommended for neutralizing and determining bactericidal activity in cosmetic products. These media include Inactivator Broth, Letheen Agar, T.A.T. Broth etc.

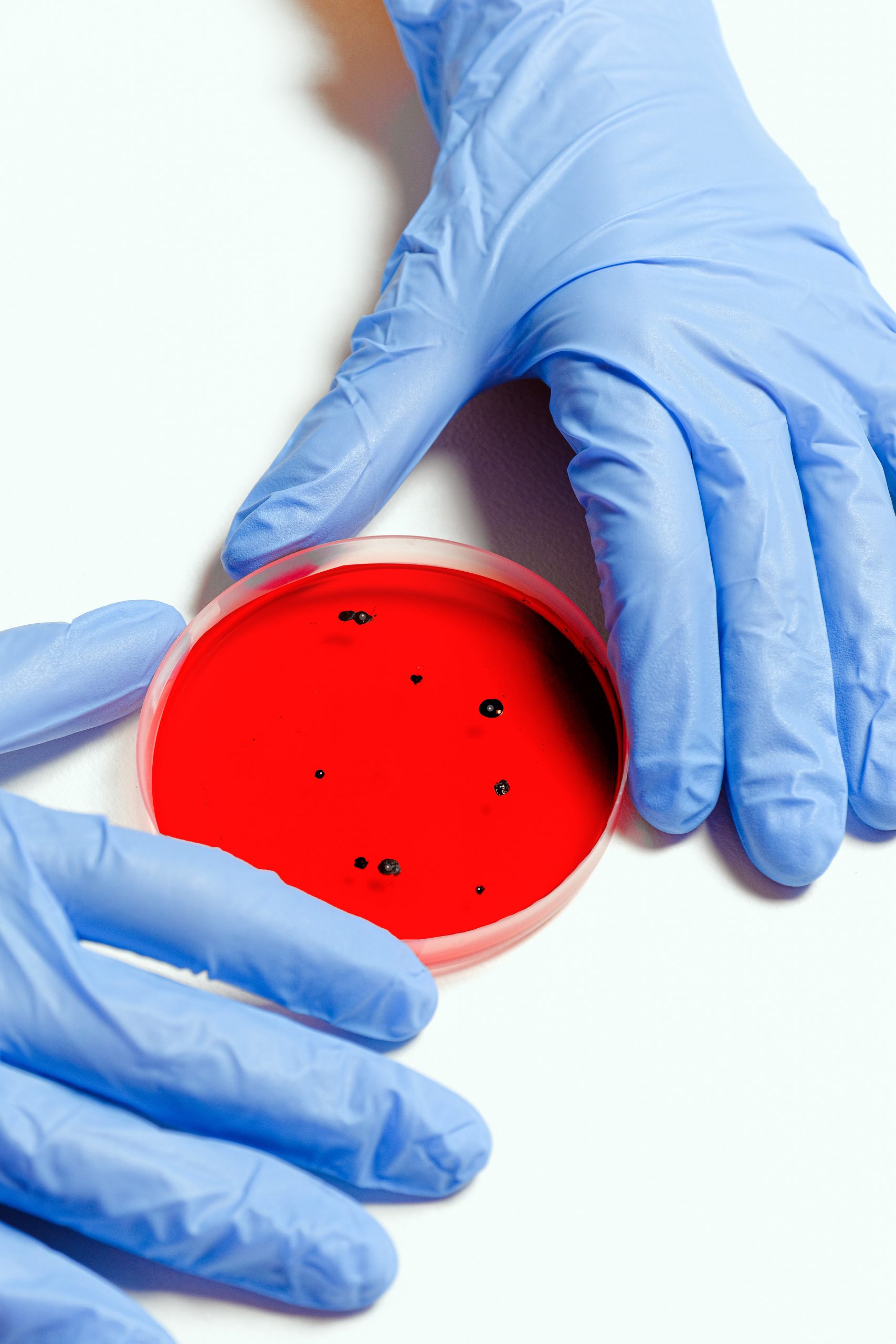
Total Microbial Count Media
Cosmetic products not only need to be good for consumer’s skin but also should not become a breeding ground for microorganisms namely bacteria and fungi. Microbes tend to form a colony or call lump once given favorable conditions and are visible on a Petri dish by the naked eye. Microbe’s damage cosmetic products while inducing odor, color changes, destabilize the emulsion etc. which in turn can damage consumers in ways like harmless itching of the skin, serious infection and even blindness if the product is used around the eyes. Tests like the APC (Aerobic Plate Count), fungal yeast test and Preservative Efficacy Test are most effective to identify microbes in cosmetics. In order to test presence and count of bacteria and fungi in cosmetics, media such as Soyabean Casein Digest Agar, Plate Count Agar, Saboraud Dextrose Agar, Saboraud Chloramphenicol Agar etc. are developed by HiMedia. Our products offer assurance to customers of quality control on their cosmetic products.

